
Lead Fluoride (PbF2)
Transmission Range
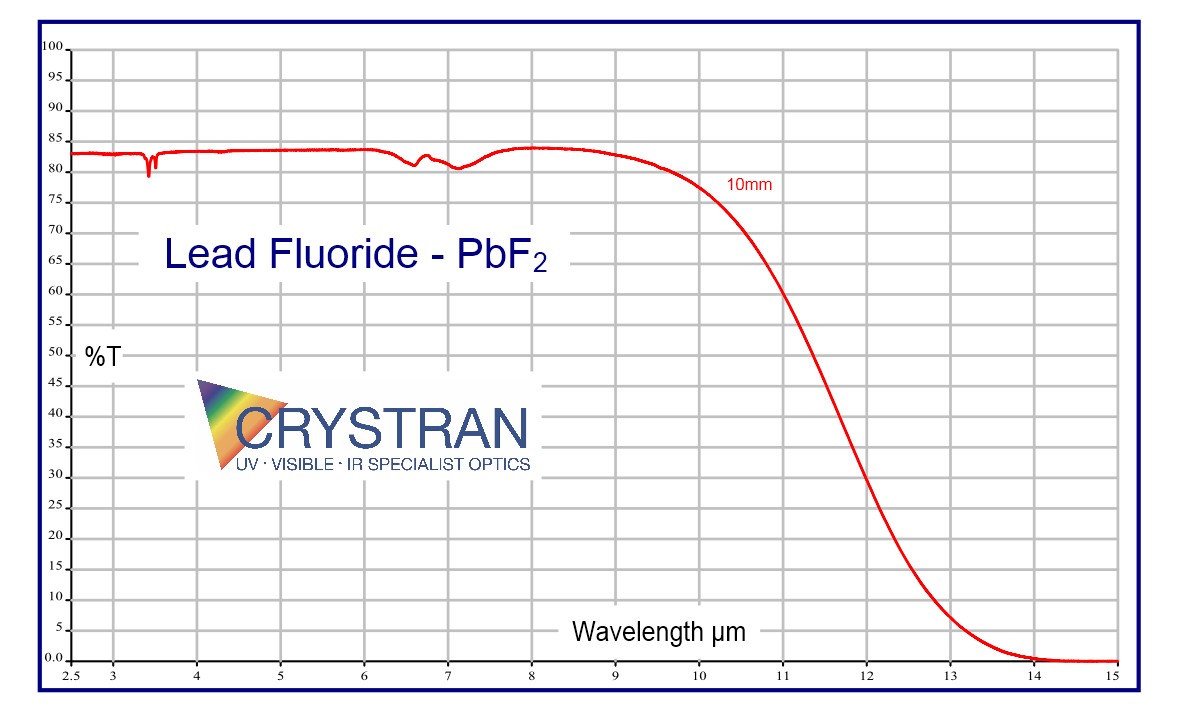
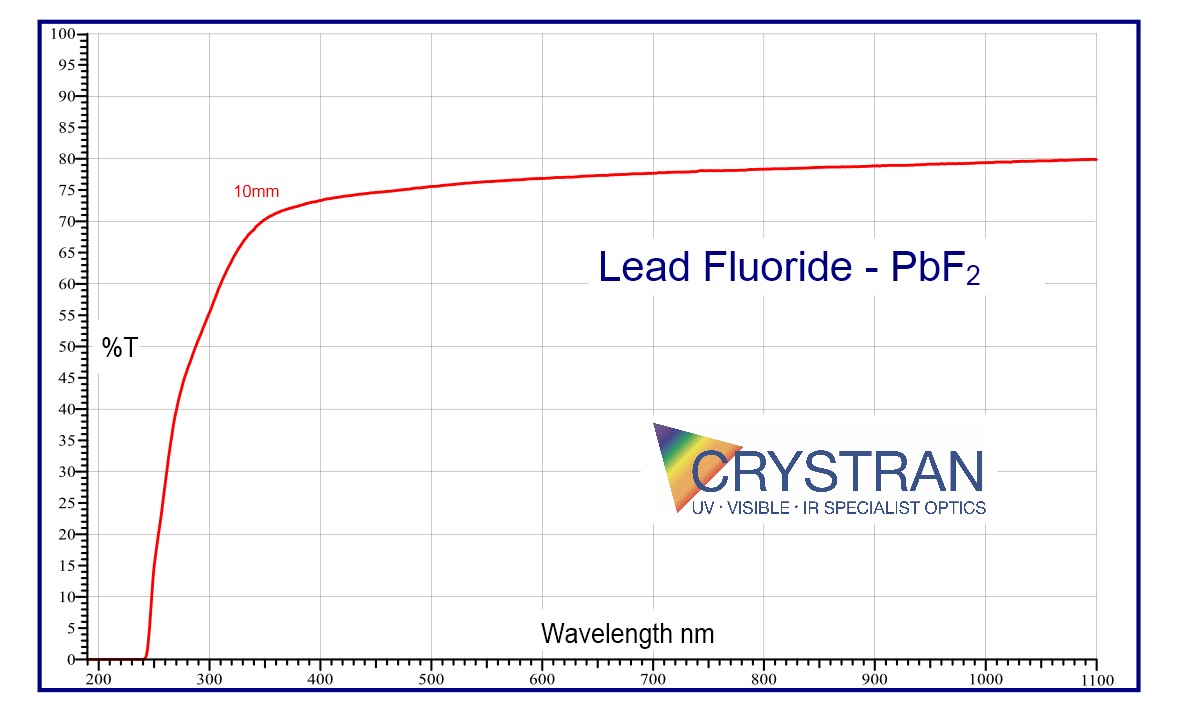
250nm to 11 μm
Refractive Index
1.7808 at 11 μm
Reflection Loss
12.8% at 5 μm (2 surfaces)
Absorption Coefficient
0.018 cm-1 @ 4μm
Reststrahlen Peak
n/a
dn/dT
n/a
dn/dμ = 0
3.3 μm
Density
7.77 g/cc (1)
Melting Point
855 °C
Thermal Conductivity
n/a
Thermal Expansion
29 x 10-6 K-1 at 283K
Hardness
Knoop 200
Specific Heat Capacity
301 J Kg-1 K-1
Dielectric Constant
13 at 1 MHz
Youngs Modulus (E)
n/a
Shear Modulus (G)
n/a
Bulk Modulus (K)
n/a
Elastic Coefficients
C11=91; C12=46; C44=23
Apparent Elastic Limit
n/a
Poisson Ratio
N/A
Solubility
0.064g/100ml water at 20°C
Molecular Weight
245.21
Class/Structure
Cubic CaF2, Fm3m, (111) cleavage
| µm | No |
|---|---|
| 0.3 | 1.93665 |
| 0.4 | 1.81804 |
| 0.5 | 1.78220 |
| 0.6 | 1.76489 |
| µm | No |
|---|---|
| 0.7 | 1.75502 |
| 0.8 | 1.74897 |
| 0.9 | 1.74455 |
| 1.0 | 1.74150 |
| µm | No |
|---|---|
| 3.0 | 1.72363 |
| 5.0 | 1.68544 |
| 7.0 | 1.68544 |
| 9.0 | 1.65504 |
Lead fluoride has been grown by vacuum Stockbarger, but is not known to be in regular production. Crystran hold a very small stock of crystal ingot.
REFERENCES
(1) Crystran Data