
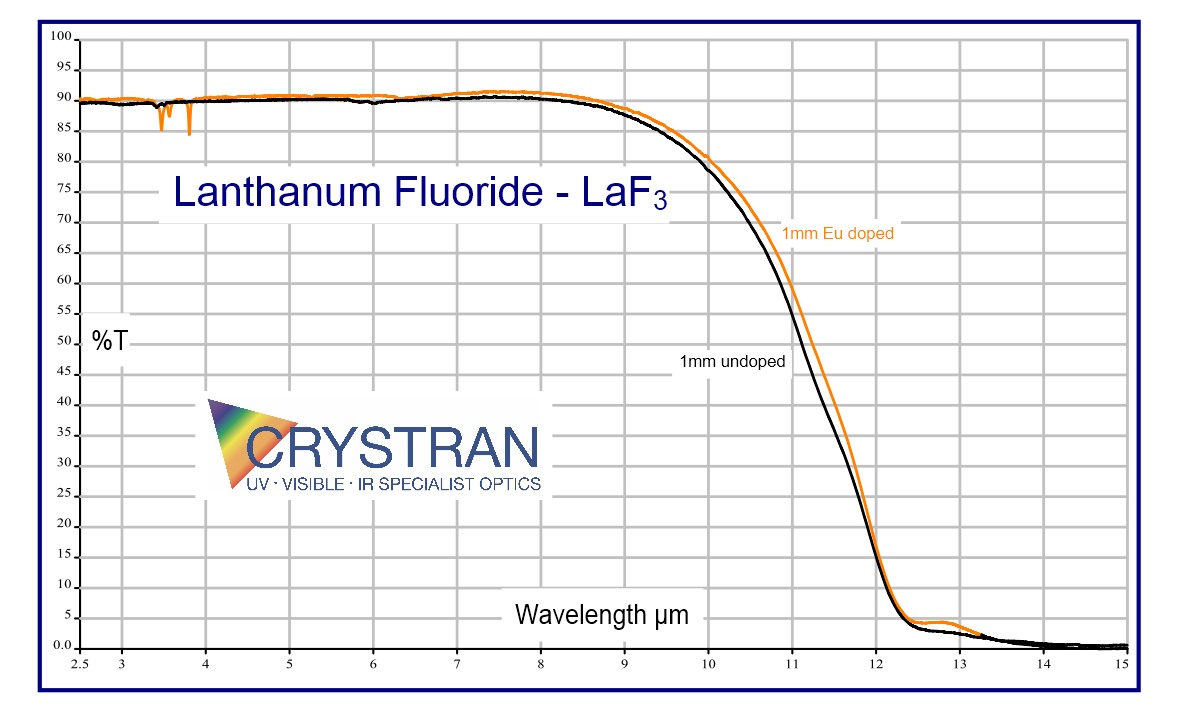
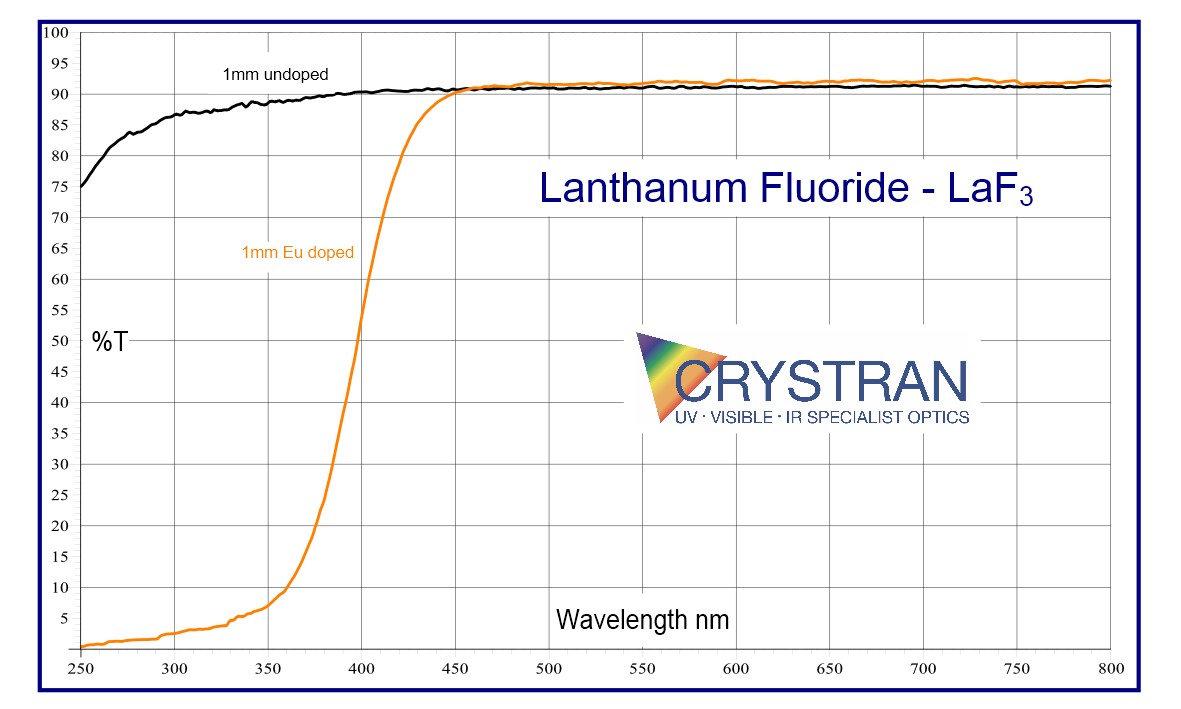
Lanthanum Fluoride LaF3:Eu3+
Lanthanum Fluoride is not often used as an optical material. Lanthanum Fluoride is usually doped with Europium at a nominal level of 0.3% mole. In this form, the usual application is as the active element in an ion-selective electrode for the detection and measurement of Fluoride ions in solution.


| µm | No | Ne |
|---|---|---|
| 0.254 | 1.656 | 1.649 |
| 0.405 | 1.618 | 1.612 |
| µm | No | Ne |
|---|---|---|
| 0.436 | 1.617 | 1.609 |
| 0.547 | 1.606 | 1.602 |
| µm | No | Ne |
|---|---|---|
Lanthanum Fluoride is grown as small ingots of about 10mm diameter as it is difficult to anneal. Doped with Europium, it is a pale yellow colour.
REFERENCES:
(1) Jones and Shand, J.Crys.Growth. 2 (1968) p3611
(2) Sher, Solomon, Lee, and Meuller. Phys.Rev. 144, p593 (1966)
(3) Electronic Processes in Ionic Crystals (OU Press, NY, 1940) p.41