
Diamond (C) Cubic Carbon
Being a form of carbon, diamond oxidises in air over 700°C. In the absence of oxygen such as in a flow or argon gas, diamond can be heated to 1700°C. The surface blackens but can be recovered by polishing.
| µm | No |
|---|---|
| 0.232 | 2.6917 |
| 0.298 | 2.5429 |
| 0.405 | 2.4626 |
| 0.589 | 2.4175 |
| 0.656 | 2.4104 |
| µm | No |
|---|---|
| 1.00 | 2.3905 |
| 2.00 | 2.3813 |
| 3.00 | 2.3795 |
| 4.00 | 2.3787 |
| 5.00 | 2.3783 |
| µm | No |
|---|---|
| 6.00 | 2.3779 |
| 10.0 | 2.3765 |
| 20.0 | 2.3741 |
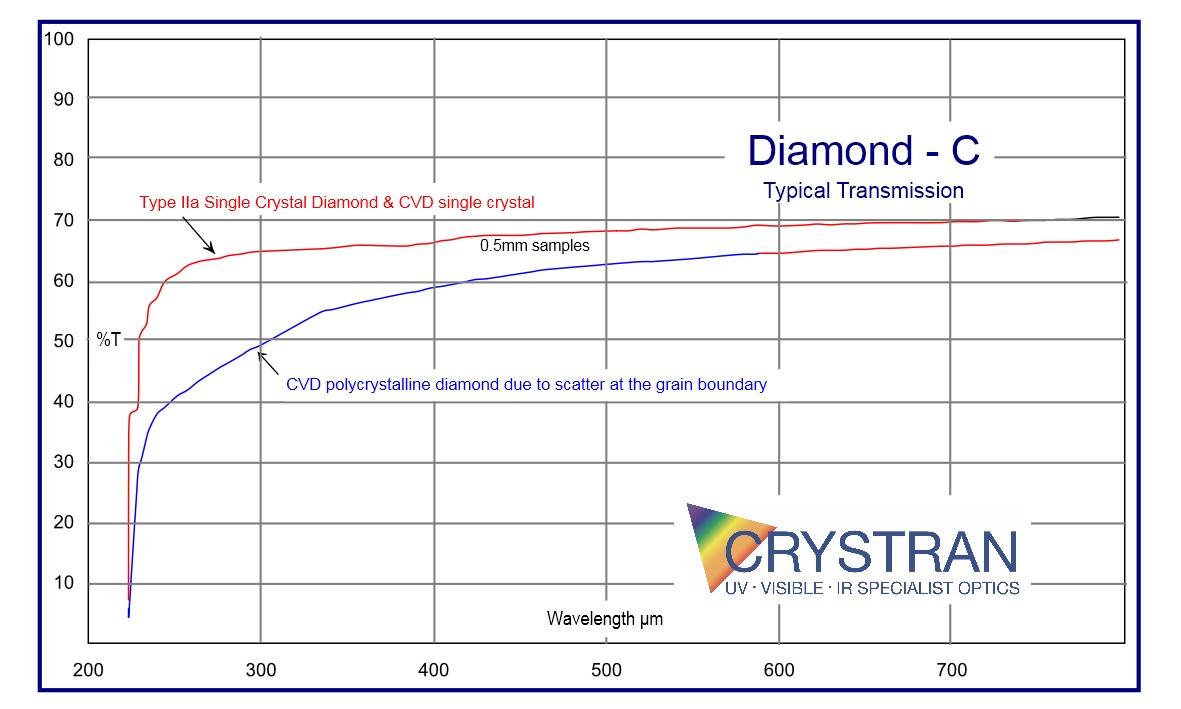
Diamond is available as single crystal (Type IIa) natural or synthetic or as polycrystalline CVD film. Single crystal is available economically up to about 4mm diameter. CVD diamond of 75mm diameter is available.
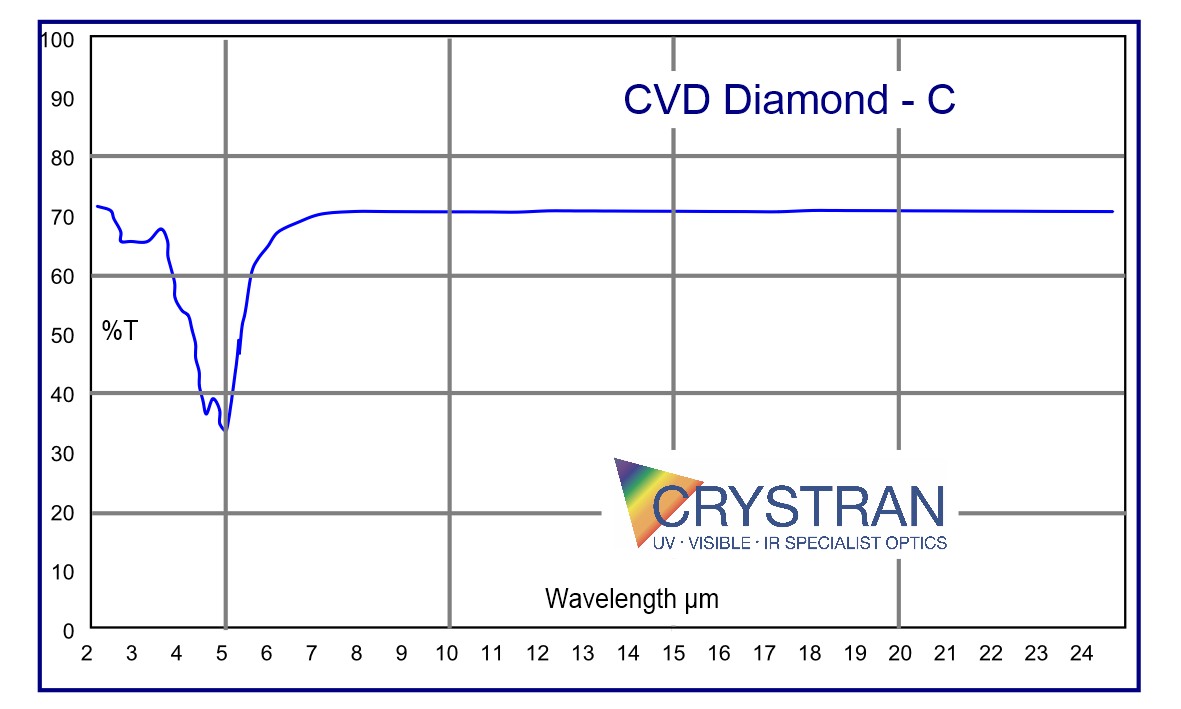
The IR transmission has a range of absorptions in the mid-IR between 2,5 and 7 microns due to inherent lattice resonances. The UV transmission may be limited to 350nm in poorer quality samples.
Single crystal diamond is selected into four forms:
Ia ~98% of natural yield (>100ppm Nitrogen - yellow)
Ib ~0.1% of natural yield (~100ppm Nitrogen)
IIa ~2% of natural yield (1ppm Nitrogen)
IIb - Synthetic only (100ppm boron for electronic applications)
Only Type IIa is used for optical applications. CVD diamond transmission and characteristics are very similar to Type IIa.
REFERENCES:
(1) Handbook Optical Constants, ed Palik, V1, ISBN 0-12-544420-6
(2) Properties Polycrystalline Diamond, Sussmann et. al. Diamond & Rel. Mat. 3(1994) 303-312
(3) Fontenella et. al. App. Optics 16, 2949 (1977)
(4) Slack & Bartram J.Appl. Phys. 46, 89 (1975)