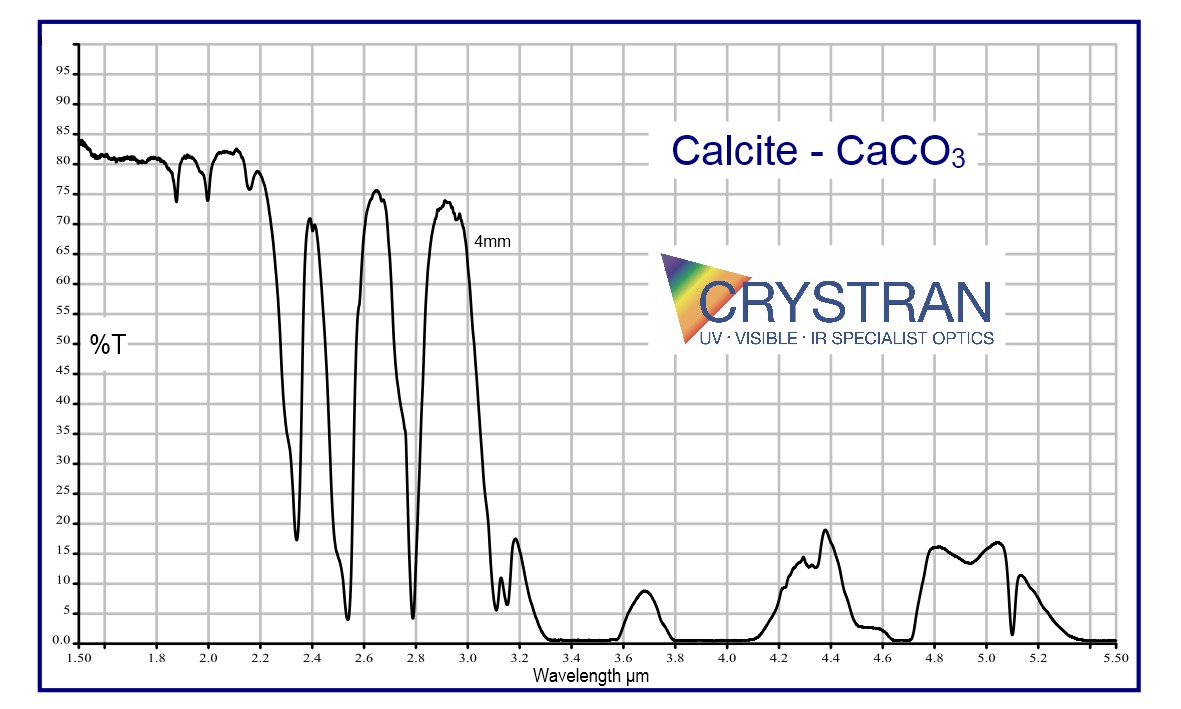

Calcite CaCO3
Transmission Range
0.3 to 2.3 μm
Refractive Index
No 1.6654 at 0.51 μm
Reflection Loss
11.7% at 0.51 μm (2 surfaces)
Absorption Coefficient
n/a
Reststrahlen Peak
n/a
dn/dT
3 (para) 13 (perp) x 10-6 K-1 at 0.5 micron
dn/dμ = 0
n/a
Density
2.71 g/cc
Melting Point
825°C (Decomposes)
Thermal Conductivity
5.526 (para) 4.646 (perp) W m-1 K-1 at 273K
Thermal Expansion
25 (para) -5.8 (perp) x 10-6 K-1 at 273K (2)(3)
Hardness
Knoop 155 Moh 3
Specific Heat Capacity
852 J Kg-1 K-1
Dielectric Constant
8 (para) 8.5 (perp) at 10kHz at 293K
Youngs Modulus (E)
72.35 (perp) 88.19 (para) GPa
Shear Modulus (G)
35 GPa
Bulk Modulus (K)
129.53 GPa
Elastic Coefficients
C11=137; C12=45; C13=45; C14=21; C33=79
Apparent Elastic Limit
4.83 MPa (700 psi)
Poisson Ratio
n/a
Solubility
0.0014g/100g water at 25°C
Molecular Weight
100.09
Class/Structure
Trigonal (hex), R3c, {10-14} cleavage (1) also see Product Notes
| µm | No | Ne |
|---|---|---|
| 0.20 | 1.9028 | 1.5765 |
| 0.30 | 1.7196 | 1.5137 |
| 0.41 | 1.6801 | 1.4954 |
| 0.51 | 1.6653 | 1.4896 |
| µm | No | Ne |
|---|---|---|
| 0.64 | 1.6550 | 1.4849 |
| 0.71 | 1.6521 | 1.4835 |
| 0.80 | 1.6487 | 1.4822 |
| 0.91 | 1.6458 | 1.4810 |
| µm | No | Ne |
|---|---|---|
| 1.04 | 1.6428 | 1.4799 |
| 1.50 | 1.6346 | 1.4774 |
| 1.91 | 1.627 | 1.4757 |
| 2.10 | 1.622 | 1.4749 |
Calcite is often mined naturally. Crystran Ltd has a stock of small calcite "rhombs" of good clear optical quality. Calcite cuts and polishes well.
CLEAVEAGE PLANE :
There can be confusion in the definition of the cleavage plane in calcite. Conventionally this has always been referred to as {10-11} but recent papers on AFM studies use {10-14}. Calcite cleaves between the bonds of the CO3 groups (in the CO3 layer). The CO3 group are offset relative to each other and inclined to the c axis giving 3 cleavage directions defining a rhomb. Following the {10-11} nomenclature the unit cell requires ¼ the length of the c axis as measured from XRD (on a dimension 4 times longer). The correct Miller indices are {10-14} but the conventional {10-11} is often used in order not to confuse and for easier comparison. (1)
REFERENCES:
1. Private Communication. J.A.Elliott. Dept. Materials Science, University of Cambridge. 2011
REFERENCES:
1. Private Communication. J.A.Elliott. Dept. Materials Science, University of Cambridge. 2011
2. Crystallography - LINK to: Practical Crystal Measurement. A.E.H.Tutton, Vol2, page 1329
3. Linear Thermal Expansion of Calcite - Link to: Mineral Society of America, Linear Thermal Expansion of Calcite.