
Barium Fluoride (BaF2)
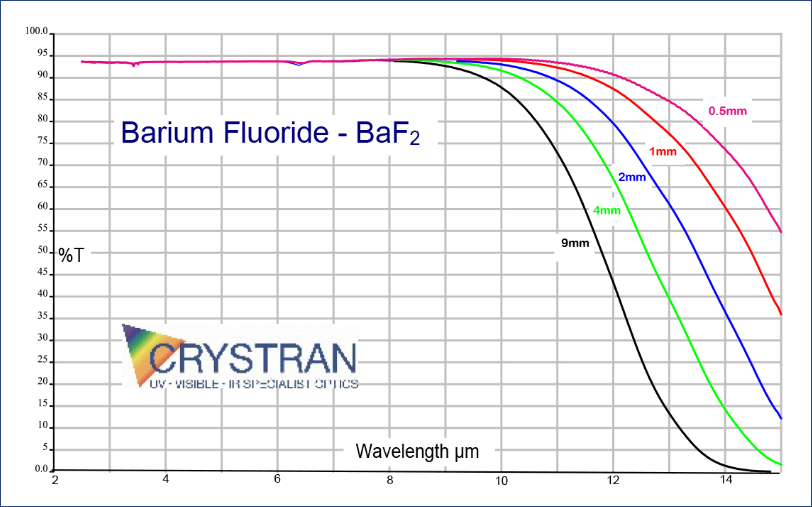
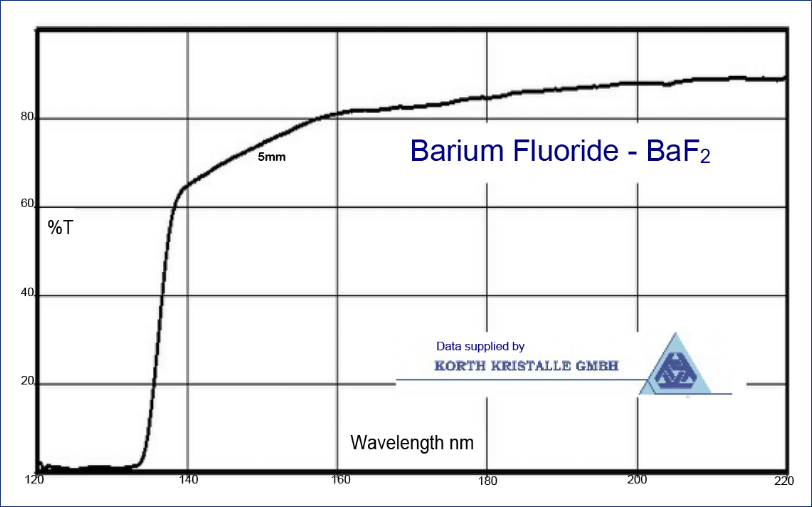
Barium Fluoride is used in spectroscopic components. Barium Fluoride is often suitable for applications in the passive IR band (8 to 14 μm) and is often used as a viewport window for thermography. For an equivalent thickness the transmission extends approximately 1 micron further into the IR than Calcium Fluoride. Windows intended primarily for IR use and noted as having IR polish may have restricted transmission in the VUV being made from lower purity BaF2 crystal.
The highest quality Barium Fluoride also has application as the fastest known scintillator material and is used in High Energy Physics Experiments.


| µm | No |
|---|---|
| 0.1408 | 1.815 |
| 0.1452 | 1.7820 |
| 0.1477 | 1.1.7670 |
| 0.1500 | 1.6780 |
| 0.2000 | 1.557 |
| 0.2652 | 1.5122 |
| 0.2803 | 1.5066 |
| 0.2893 | 1.5039 |
| 0.2967 | 1.5019 |
| 0.3021 | 1.500 |
| 0.3130 | 1.4978 |
| 0.3254 | 1.4952 |
| 0.3403 | 1.4925 |
| 0.3466 | 1.4915 |
| 0.3610 | 1.4894 |
| µm | No |
|---|---|
| 0.3663 | 1.4887 |
| 0.4046 | 1.4844 |
| 0.5461 | 1.4759 |
| 0.5893 | 1.4744 |
| 0.6438 | 1.4730 |
| 0.6563 | 1.4727 |
| 0.7065 | 1.4718 |
| 0.8521 | 1.4699 |
| 0.8944 | 1.4694 |
| 1.0140 | 1.4685 |
| 1.1287 | 1.4678 |
| 1.3673 | 1.4667 |
| 1.5295 | 1.4661 |
| 1.6810 | 1.4656 |
| µm | No |
|---|---|
| 1.7012 | 1.4655 |
| 1.9701 | 1.4647 |
| 2.3254 | 1.4636 |
| 2.6738 | 1.4623 |
| 3.2434 | 1.4602 |
| 3.4220 | 1.4594 |
| 5.1380 | 1.4501 |
| 5.5490 | 1.4473 |
| 6.2380 | 1.4422 |
| 6.6331 | 1.4390 |
| 7.0442 | 1.4353 |
| 7.2680 | 1.4331 |
| 9.7240 | 1.4051 |
| 10.346 | 1.3936 |
Barium Fluoride is grown by vacuum Stockbarger technique. Unlike CaF2, BaF2 is not found in the native state and all material must be synthesised chemically making BaF2 relatively expensive to produce. Barium Fluoride cleaves easily and is highly susceptible to thermal shock. It polishes well and can be etched (5). The highest purity Barium Fluoride VUV material can be qualified as fast scintillator grade. (6)
The highest purity Barium Fluoride VUV material can be qualified as fast scintillator grade. (6)
REFERENCES:
(1) Handbook Optical Constants, ed Palik, V3, ISBN 0-12-544423-0
(2) I.H.Malitson; J.Opt.Soc.Am. Vol52, p1377, 1962
(3) D.Girlich; Elastic Constants of BaF2; Phys.Rev. Vol135, p1826, 1964
(4) S.Ballard et al; J.Opt.Soc.Am. Vol42, p684, 1952
(5) US patent. Chemical polish. 4,040,896 1977
(6) M.Laval et al; Nu. Insts.Meth, V206 p169, 1983